Original link:Transformation of viral vectors in metaorganisms
The main research directions of basic R&D currently include lentivirus (LV) vector modification, adenovirus (ADV) capacity modification, adenovirus (AAV) serotype screening, specific promoter optimization, comprehensive development of CRISPR/Cas9 technology with in vivo application as the core, and the development of other biological research tools.
The viral vector is a key link in achieving gene delivery and completing gene manipulation, therefore, the modification of viral vectors plays a special and important role in research and application. The modification of virus vectors by the R&D department of Heyuan mainly includes the in vivo application modification of adenovirus, including the modification of dozens of targeted specific organ serotypes, various types of AAV serotype libraries, lentivirus and adenovirus.
The commonly used rAAV in current research is a hybrid viral vector produced by combining AAV2 genome with different capsid proteins, generally labeled as rAAV2/N (where N represents different capsid serotypes). The recombinant virus has stable expression and gene integration ability of AAV2 type, while obtaining tissue infection affinity for different serotypes (specific structural sites on the surface of the capsid of different serotypes determine the specificity of their respective receptors), exhibiting certain organ targeting specificity.
Tissue hydrophilicity of AAV with different serotypes
Serotype | Organizational affinity |
rAAV2/1 | The nervous system (high titer forward synaptic), Muscle, skeletal muscle, myocardium, smooth muscle |
rAAV2/2 | Nervous system, muscles, liver, Smooth muscle of blood vessels, eye |
rAAV2/3 | Muscles, liver, lungs, eyes |
rAAV2/4 | Nervous system, muscles, eyes, brain |
rAAV2/5 | The nervous system, lungs, retina, Liver, synovial joint |
rAAV2/6 | Nervous system, lungs, muscles, heart |
rAAV2/7 | Muscle, liver |
rAAV2/8 | Nervous system, liver, pancreas, Retina, adipose tissue |
rAAV2/9 | Nervous system, myocardium, lungs, Retina, skin, muscle, adipose tissue |
rAAV2-retro | The nervous system (reverse non synaptic) |
AAV-PHP.eB | Cross blood-brain barrier (intravenous injection) |
AAV-PHP.S | Whole peripheral nerve (tail vein injection) |
AAV-PAN | Pancreatic (intraperitoneal injection) |
AAV-LUNG | Lung (tail vein injection) |
AAV-DJ | Retina, lungs, kidneys, Infection of cells in vitro |
AAV-7m8 | retina |
AAV-ShH10Y | Muller cells in the retina |
AAV-Rh10 | Liver, blood, heart, Infection of cells in vitro |
AAV-Anc80L65 | Inner ear, retina、 Skeletal muscle, liver |
AAV-SCH9 | SVZ neural stem cells |
AAV-2M | retina |
AAV-BR1 | Cerebrovascular endothelial cells |
AAV6-TM6 | Microglia |
Targeted specific organ AAV serotype infection case
1. RAAV2 retro: nervous system (reverse non synaptic)
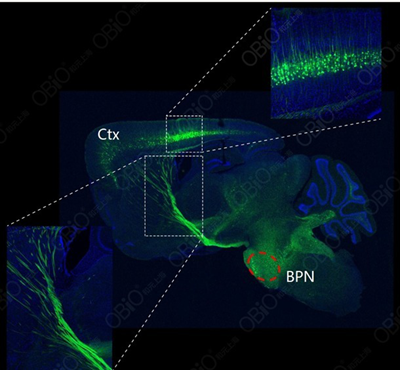
Injection site: Mouse BPN
Injection method: Brain localization injection
Carrier: rAAV2-retro hSyn-EYFP
Observation time: 4 weeks
2. AAV-PHP-eB: Whole brain expression of the nervous system (across the blood-brain barrier)
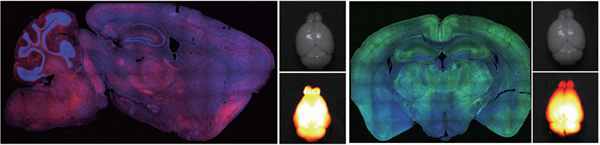
Injection method: Tail vein injection
Carrier: pAAV-CMV-mScarlet-3FLAG&pAAV-CMV-mNeonGreen-3FLAG
Serotype: AAV-PHP-eB
Virus titers: 1.10 × 1013VG/mL&2.35 × 1013VG/mL
Injection volume: 200nl (total amount of injected virus: 1.5 × 1011 VG)
Observation time: 3 weeks
3. AAV-PAN: Specific infection of pancreas
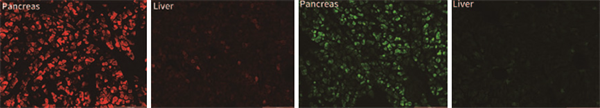
4. AAV-LUNG: Specific infection of the lungs

Injection method: Tail vein injection Vehicle: pAAV-CMV-mScarlet-3FLAG
Serotype: AAV-LUNG
Injection volume: 2x1011 VG/animal (titer: 1.49 x1013 VG/mL),
Injection volume: 200 uL
Observation time: 3 weeks
5. AAV-DJ infection of 293T cells in vitro

AAV-DJ(MOI=105) AAV8(MOI=105 )






 Back
Back





















