Original link:Meta organism - Adeno associated virus AAV
Adeno associated virus (AAV) is a member of the parvoviridae family, which is a type of icosahedral parvovirus that cannot replicate autonomously and has no membrane. It has a diameter of about 20-26nm and contains approximately 4.7kb of linear single stranded DNA as its genome. The recombinant adeno-associated virus (rAAV) vector used in the study is a gene vector modified from non pathogenic wild-type AAV. Due to its diverse types, extremely low immunogenicity, high safety, wide host cell range (infectivity to both dividing and non dividing cells), strong spreading ability, and long in vivo gene expression time, rAAV is considered one of the most promising gene research and gene therapy vectors.
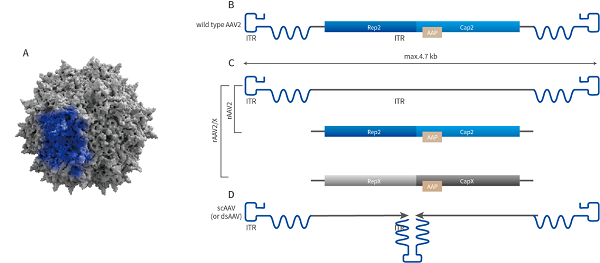
AAV genome structure
RAAV packaging purification and titer detection
During the AAV packaging process, the packaging plasmid is responsible for encoding the target gene and two terminal reverse repeat sequences (ITR), which play a decisive role in virus replication and packaging. The auxiliary plasmid contains the cap (encoding viral capsid protein) and rep (involved in virus replication) genes required for AAV packaging, as well as the adenovirus helper plasmid. After co transfection of three plasmids into 293T cells, AAV virus begins to replicate and package. The production and quality control of adenovirus associated with metaorganisms follow internationally recognized standard procedures and are packaged in 293T cells using a three plasmid system. The obtained virus particles were purified by ultracentrifugation and the virus gene copies were titrated by qPCR. In addition, we can also provide protein staining to detect the integrity of capsid proteins and endotoxin content according to user requirements. In general, the titer of rAAV is between 1012 and 1013 VG/ml, which can fully meet the requirements of various overall experiments.
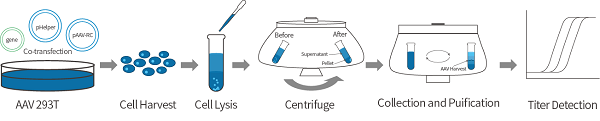
AAV Packaging Process Diagram
Titer detection method: Quantitative PCR is used to detect the copy number of exogenous DNA in the viral genome.
Titer detection principle: The genome of adenovirus is single stranded DNA, and the copy number of exogenous DNA represents the copy number of the virus genome.
Process of rAAV Infection into Cells
The purified AAV viral vector can be used to infect cells. When infecting cells, AAV binds to specific receptors on the cell surface, activates intracellular signaling pathways, and triggers AAV to enter the cell through receptor-mediated endocytosis. With the assistance of organelles such as the nucleus and Golgi apparatus, AAV enters the nucleus and then the virus lyses. Its single stranded DNA needs to replicate into double stranded DNA to express the target gene.
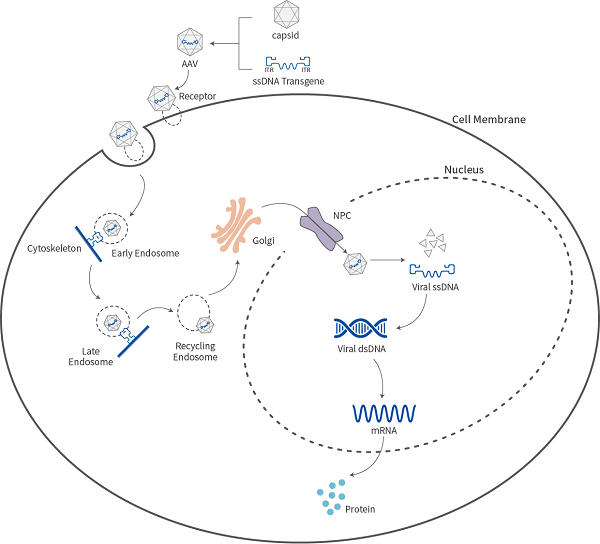
Mechanism of rAAV action
Advantages of rAAV Carrier
1. High safety and low immunogenicity: AAV is a replication defective DNA virus with no autonomous replication ability. Wild type AAV relies on the rep gene for low-frequency site integration, while rAAV does not integrate. At present, there are no reports of human and mammalian diseases caused by AAV, and it is also one of the safest viral vectors approved by the FDA for gene therapy drugs.
2. Wide host cell range: AAV has a wide host range and has the ability to infect both dividing and non dividing cells.
3. Strong diffusion ability: AAV has a diameter of about 20-26nm, a small volume, high titer, and good diffusion ability. Among them, AAV-PHP.EB and AAV9 have the ability to cross the blood-brain barrier and are widely used in the field of neuroscience.
4. Long duration of gene expression in vivo: AAV has the ability to maintain long-term gene transcription expression, with peak expression typically occurring within 3 weeks and sustained high expression for more than 5 months.
5. Diverse types: AAV serotypes are numerous, and in addition, we are constantly mutating and screening for new serotypes (AAV1-13, AAV2/1, 2/2, 2/5, 2/6, 2/8, 2/9, DJ; retro, PHP.eB…)。
Comparison of Biological Characteristics of Different Viruses
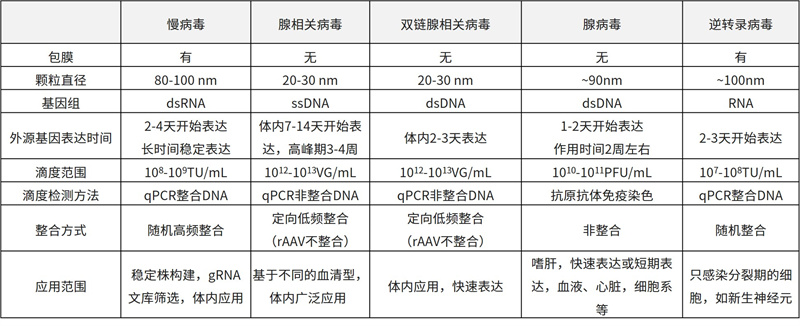
Based on the above characteristics, AAV is considered an efficient and safe in vitro and in vivo gene transduction tool. Especially in overall level research, compared with other commonly used viral tool vectors, AAV has a mild and long-lasting expression ability during the infection process, making it a powerful tool for gene manipulation.
RAAV serotype selection
At present, the total number of registered AAV species has exceeded 196, and there are 13 different serotypes of AAV (i.e. AAV1-AAV13) in primates. Among them, AAV2, AAV3, and AAV9 originate from humans themselves. It is worth noting that AAV2 is the earliest cloned virus and the most thoroughly studied and widely used viral vector to date. With the continuous deepening of research, researchers have found that AAV of different serotypes can hybridize, and the hybridized AAV will have the characteristics of both heterozygotes. Therefore, AAV subtypes are born naturally.
The commonly used rAAV in current research is a hybrid viral vector produced by combining AAV2 genome with different capsid proteins, generally labeled as rAAV2/N (where N represents different capsid serotypes). The recombinant virus has stable expression and gene integration ability of AAV2 type, while obtaining tissue infection affinity for different serotypes (specific structural sites on the surface of the capsid of different serotypes determine the specificity of their respective receptors), exhibiting certain organ targeting specificity.
Tissue hydrophilicity of AAV with different serotypes
Serotype | Organizational affinity |
rAAV2/1 | The nervous system (high titer forward synaptic), muscles, Skeletal muscle, cardiac muscle, smooth muscle |
rAAV2/2 | Retina, nervous system, Muscle, liver, vascular smooth muscle |
rAAV2/3 | Muscles, liver, lungs, eyes |
rAAV2/4 | Nervous system, muscles, eyes, brain |
rAAV2/5 | The nervous system, lungs, retina,, Liver, synovial joint |
rAAV2/6 | The nervous system, lungs, Muscle, heart |
rAAV2/7 | Muscle, liver |
rAAV2/8 | The nervous system, liver, muscles, Adipose tissue, pancreas, retina, |
rAAV2/9 | Nervous system, myocardium, lungs, Retina, skin |
rAAV2-retro | The nervous system (reverse non synaptic) |
AAV-PHP.eB | Cross blood-brain barrier (intravenous injection) |
AAV-PHP.S | Whole peripheral nerve (tail vein injection) |
AAV-PAN | Pancreatic (intraperitoneal injection) |
AAV-LUNG | Lung (tail vein injection) |
AAV-DJ | Retina, lungs, kidneys, Infection of cells in vitro |
AAV-7m8 | retina |
AAV-ShH10Y | Muller cells in the retina |
AAV-Rh10 | Liver, blood, heart, Infection of cells in vitro |
AAV-Anc80L65 | Inner ear, retina、 Skeletal muscle, liver |
AAV-SCH9 | SVZ neural stem cells |
Small suggestion: If you are unsure about which serotype to choose, you can try conducting pre experiments with Pandora's Virus (AAV serotypes) and compare the infection effects of different serotypes on the target tissue to explore the best experimental conditions (injection method, injection site, virus dosage, etc.), in order to obtain more ideal experimental results.
Pandora's Virus Details:
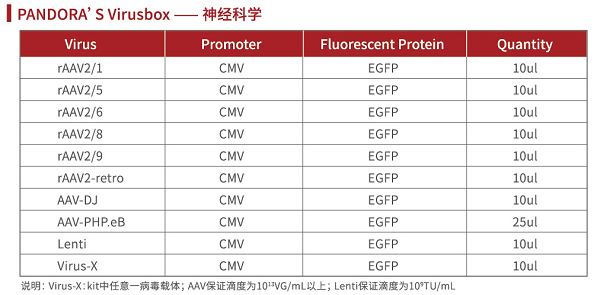

Application case of rAAV carrier
1. Applications in the field of neuroscience
1.1 Lateral habenular nucleus&optogenetics
Customer published article: Nature (IF=41.577). Yang Y,et.al. (2018). Ketamine blocks burning in the lateral habitua to rapidly relieve depression
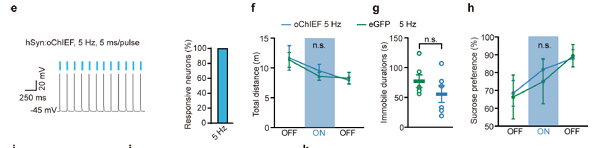
Injection site: Mouse LHb
Vector: AAV2/9-hSyn-oChIEF tdTomato
Serotype: AAV2/9
Virus titer: 6.29E+12 VG/mL
Injection volume: 100nl
Observation time: 1 month
1.2 Brachial Nucleus&Chemical Genetics
Customer published article: Science (IF=41.058). Mu D,et.al. (2017). A central neural circuit for itch sensing

Injection site: Mouse PBN
Vector: AAV hSyn HA hM4Di IRES mCitrine
Serotype: AAV2/9
Virus titer: 1E+13 VG/mL
Injection volume: 150nl
Observation time: 3 weeks
1.3 Thalamus and Calcium Ion Imaging
Customer article: Customer article: Science (IF=41.058). Ren S,et.al. (2018). The paraventricular thalamus is a critical thalamic area for wakefulness

Injection site: Mouse PVT
Vector: AAV CaMKII α - GCaMP6f
Injection volume: 100nl
Observation time: 4 weeks
1.4 Periventricular nucleus of thalamus&killing neurons
Customer published article: Science (IF=41.058). Ren S,et.al. (2018). The paraventricular thalamus is a critical thalamic area for wakefulness
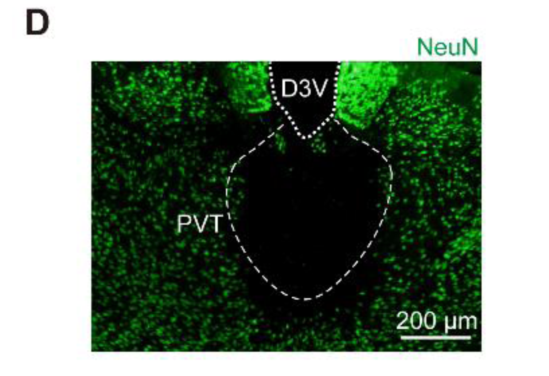
Injection site: Mouse PVT
Vector: AAV-CaMKII α - Cre GFP&AAV-DIO-caspase-3
Injection volume: Mix two viruses 1:1 and inject 100nl
Observation time: 4 weeks
1.5 Hippocampus, cortex&retrograde non synaptic (rAAV2/retro)
Customer published article: Biological Psychiatry (IF=11.984). Bing Xing Pan,et.al. (2018). Chronic stress causes projection specific adaptation of amygdala neurons via SK channel downregulation
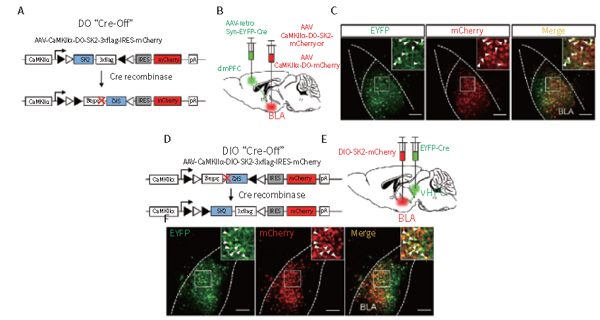
Injection site: mouse dmPFC, VHPC
Vector: AAV2/1-reco-Syn-eYFP Cre AAV2/8-CaMKIIα-DO-SK2&AAV2/8-CaMKIIα-DIO-SK2-mCherry
Injection volume: 300nl
Observation time: 4 weeks
1.6 Primary motor cortex&anterograde synapse (rAAV2/1)
Nature Neuroscience. (IF=19.912). Yao J,et.al. (2018). A corticopontine circuit for initiation of urination. [Adeno associated virus, neural circuits]
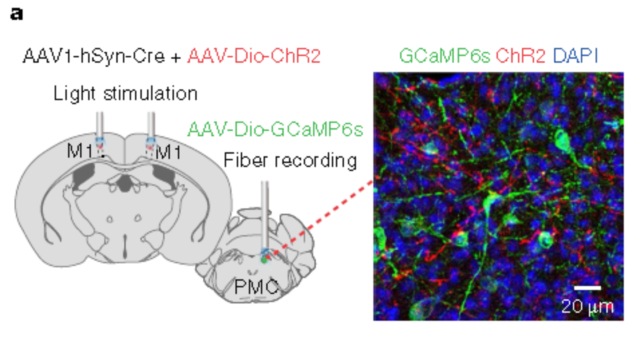
Injection site: Mouse cortex M1, PMC
Carrier: rAAV2/1-hSyn-Cre&rAAV2/9-DIO-hChR2 (H134R) - mCherry, rAAV2/9-DIO-GCaMP6s
Virus titer: rAAV2/1: 5E+12 VG/mL; rAAV2/9-DIO-hChR2:1.2E+13 VG/mL;rAAV2/9-DIO-GCaMP6s:1E+12 VG/mL
Injection volume: multi-point injection, 30-40 ml per point
Observation time: 4 weeks
1.7 Edge prefrontal cortex&cross synaptic tracing (WGA Cre)
Customer published article: Science Advances (IF=11.511). Ping Zheng,et.al. (2019). Critical role of feedback signals from prellimbic cortex to basolatoral amygdala in the retrieval of morphological withdrawal memory. [Adeno associated virus, addiction, AAV1, AAV-WGA Cre]
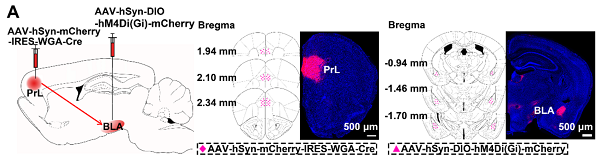
Injection site: Mouse PrL
Vector: AAV hSyn mCherry IRES WGA Cr
Virus titer: 4.28E+12 VG/mL
Injection volume: 300nl
Observation time: 4 weeks
1.8 Cortical and AAV Vector (AAV shRNA)
Customer posts articles:Nature Medicine. (IF=22.864). Cao X,et.al. (2013). Astrocyte-derived ATP modulates depressive-like behaviors.[Adeno associated virus, depression]
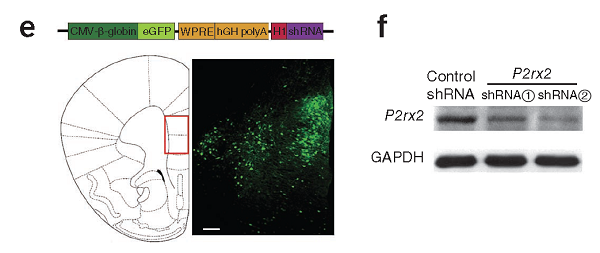
Injection site: Mouse mPFC
Vector: AAV-CMV-P2rx2 shRNA EGFP
Virus titer: 3E+12 VG/mL
Injection volume: 1.5ul
Observation time: 2 weeks
1.9 Whole Brain Expression&Cross Blood Brain Barrier (AAV-PHP. eb)
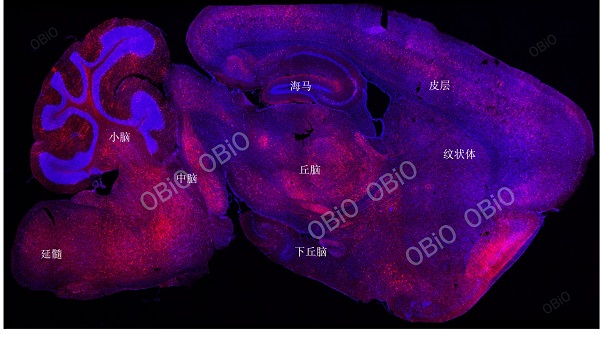
An example of whole brain infection caused by tail vein injection of rAAV-PHP-eB in He Yuan Biotech
Total virus load: 1.5E+11VG
Injection volume: 200ul
Virus expression time: Four weeks
1.10 Sparse Marking
Application Literature:Luo WS, et al., (2016) Supernova: A Versatile Vector System for Single Cell Labeling and Gene Function Studies in vivo. Sci Rep.
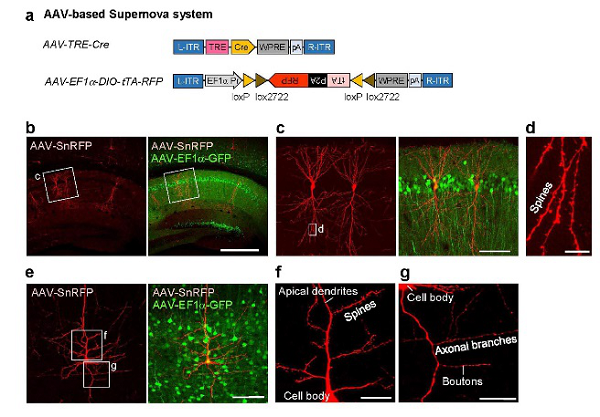
Carrier information:pAAV-PTRE-tight-NLS-Cre(108-109VG/mL)、pAAV-EF1a-DIO(tTA-P2A-mScarlet)-WPRE(1012VG/mL)
1.11 Application in the retina
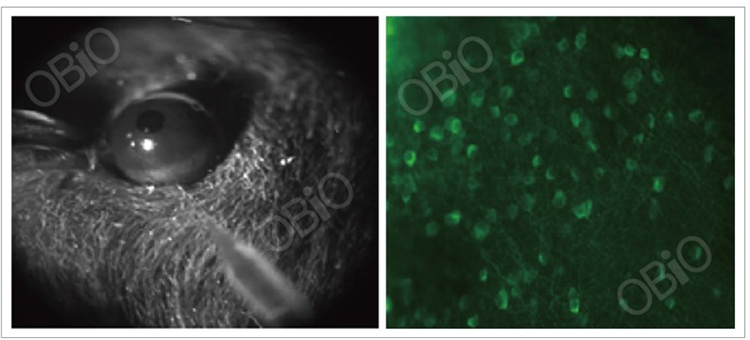
Hemibiome - intravitreal injection
Viral vector:AAV-syn-GcaMP6s
2. Application in other organs
2.1 rAAV infection of the liver
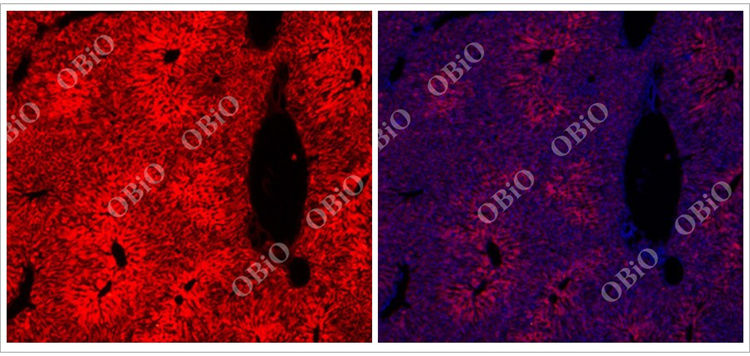
An example of liver infection caused by tail vein injection of rAAV2/8 in He Yuan Biotechnology
Total virus load: 1E+11VG
Injection volume: 200ul
Virus expression time: Four weeks
2.2 rAAV infection of the heart
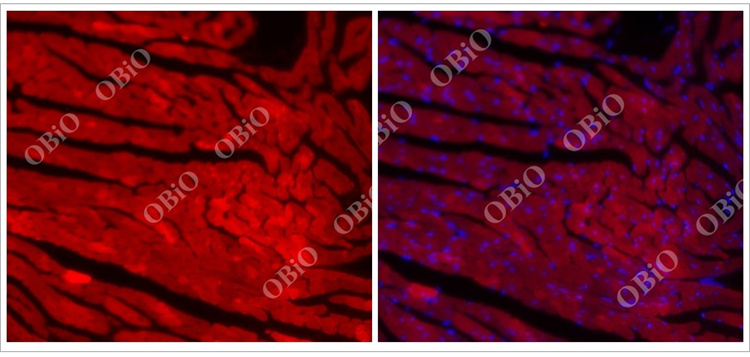
An example of cardiac infection caused by tail vein injection of rAAV2/9 in He Yuan Biotech
Total virus load: 2E+11VG
Injection volume: 200ul
Virus expression time: Four weeks
2.3 rAAV infection in the lungs

An example of pulmonary infection caused by tail vein injection of rAAV Lung in He Yuan Biotech
Total virus load: 5E+11VG
Injection volume: 200ul
Virus expression time: Two weeks
Customer posts articles:Cell. (IF=36.216). Huijuan Wu,et.al. (2020). Progressive Pulmonary Fibrosis Is Caused by Elevated Mechanical Tension on Alveolar Stem Cells. [Adeno associated virus, interference]

Injection method: Tracheal injection
Infection site: lungs
Vector: pAAV-Tgfb1 shRNA
Serotype: rAAV2/9
Virus injection volume: 1E+11 VG (50ul)
Observation time: 3 weeks
2.4 rAAV infection of kidneys

An example of renal infection caused by tail vein injection of rAAV2/8 in He Yuan Biotechnology
Total virus load: 2E+11VG
Injection volume: 200ul
Virus expression time: Four weeks
2.5 rAAV infection of adipose tissue
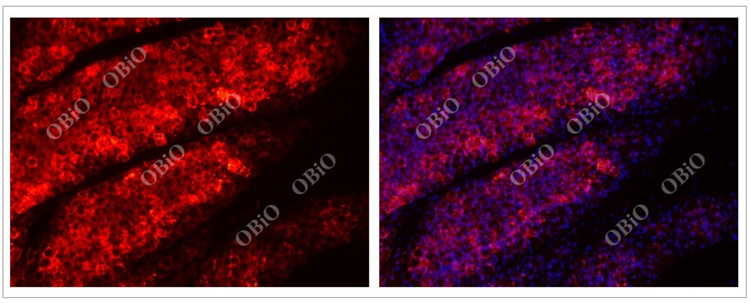
Example of rAAV2/8 inguinal fat multi-point injection infection with adipose tissue from He Yuan Biotechnology
Total virus load: 4E+10VG/side
Virus expression time: Four weeks
2.6 rAAV infection in muscle tissue

Example of Multi point Injection Infection of Muscle in Rear Legs by rAAV2/8 in He Yuan Biotech
Total virus count: 4E+10/side
Virus expression time: Four weeks
2.7 rAAV infection of pancreas
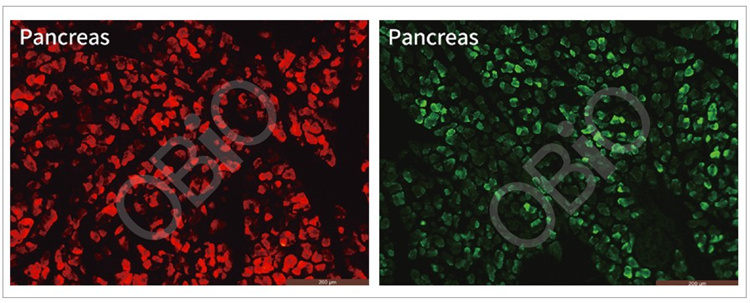
Example of intraperitoneal injection of rAAV PAN into the pancreas by He Yuan Biotech
Total virus load: 4E+11VG
Expression testing time: 4 weeks






 Back
Back





















